IT’S (NOT) MAGIC – GENETICALLY-MODIFYING MALARIA-CARRYING MOSQUITOES


source: CDC
BY EMMA ELLIS – Abracadabra. The word conjures images of long capes and studded wands, perhaps even of a furry bunny peeking its head out from a black top hat. It precedes the reveal of a woman’s body, cut in half and then put back together, or the never-ending handkerchief, forever falling out of the magician’s pocket. Yet, the word actually originates from the disease that took 445,000 lives in 2016, the majority of which were children under the age of five. Abracadabra: the phrase written on amulets of infected Romans during the end of the Roman Empire. Just like the magician in your kindergarten magic show, they too were hoping for magic. However, in their case, they were longing for some mystical force that would allow them to survive the simultaneous fevers and chills that had taken over their bodies.
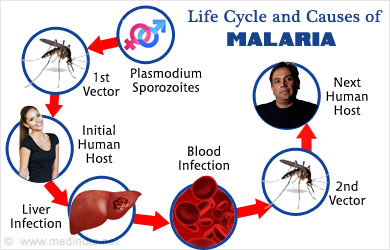
Malaria has been present in the world for many generations, but it was not until 1898 that its mode of transportation was realized. Essentially, mosquitoes become infected with malaria because of a parasite living in their stomachs. Not only do mosquitoes spread malaria by biting humans and thereby transmitting the disease into their blood, but they too can become infected by biting a human who already has malaria. Thus, the two processes stimulate each other, continuing the spread of the disease. The CDC qualifies typical symptoms of malaria as fevers, chills, and flu-like symptoms, which can be debilitating even if the disease is not fatal.
However, new forms of controlling the disease beyond bed nets and insecticides have recently come to light, namely genetically-modifying the mosquitoes using the recent CRISPR-Cas9 method. Doudna of the University of Berkley and Charpentier of Umea University are credited with utilizing a process already used to target viruses in bacteria to create CRISPR, a cheaper, more precise tool for editing genes. CRISPR is able to modify a single organism, but when only implemented in somatic cells, or those not involved in reproduction, these modifications will not affect an entire population. However, this limitation is largely solved by pairing CRISPR with gene drives, “selfish genes” which are able to increase their presence in an organism’s gametes to improve the likelihood of being passed onto future generations. Although scientists have been familiar with gene drives since 1887, they have been unable to utilize its power until the development of CRISPR technology.
At this point in time, there are two prevailing plans proposed to curb the spread of malaria. The first plan is to make mosquitoes increasingly resistant to the parasite that causes malaria. From John Hopkins School of Public Health, George Dimopoulos led an initial study in which he noted that deleting the FREP1 gene from Anopheles gambiae mosquitoes decreased the likelihood of parasites surviving and reproducing in mosquitoes, thereby decreasing the amount of parasites in the salivary glands of the mosquitoes. Dimopoulos concludes that the mosquitoes’ ability to transmit malaria to humans was significantly minimized through this modification, which would lead to a decline in the insect’s ability to transmit malaria. By combining deletion of FREP1 gene with a gene drive, this mutation could quickly spread throughout the Anopheles gambiae species, credited for most often transmitting malaria. However, the process needs to be fine-tuned before reaching that point, as mosquitoes with an inactivated FREP1 gene also saw fitness costs, suggesting they would not necessarily compete well with normal mosquitoes.
 The second plan, spearheaded by the London school, is to decrease the number of mosquitoes present in a community rather than trying to immunize all species. This approach would target three of approximately 3,500 mosquito species in the world–Anopheles gambiae, Anopheles coluzzii, and Anopheles arabiensis–by increasing the prevalence of genes that increase female infertility or sex ratios that favor males. In September, the London school revealed that their genetic modification, when combined with a gene drive, was present in all mosquitoes in their lab after just 7-11 generations, illustrating the ability to spread this modified gene across an entire species. Although this may initially seem ecologically concerning, Kayondo, the senior researcher at the Uganda Virus Research Institute, points out that no predator feeds solely on mosquitoes, and many other species that do not carry the parasite will continue to exist in the ecosystem.
The second plan, spearheaded by the London school, is to decrease the number of mosquitoes present in a community rather than trying to immunize all species. This approach would target three of approximately 3,500 mosquito species in the world–Anopheles gambiae, Anopheles coluzzii, and Anopheles arabiensis–by increasing the prevalence of genes that increase female infertility or sex ratios that favor males. In September, the London school revealed that their genetic modification, when combined with a gene drive, was present in all mosquitoes in their lab after just 7-11 generations, illustrating the ability to spread this modified gene across an entire species. Although this may initially seem ecologically concerning, Kayondo, the senior researcher at the Uganda Virus Research Institute, points out that no predator feeds solely on mosquitoes, and many other species that do not carry the parasite will continue to exist in the ecosystem.
While the technology is increasingly becoming accessible to deploy in the field, the next step is partnering with the regions in which these genetically-modified mosquitoes will be released. Target Malaria, one of Bill and Melinda Gates’ partner organizations, has developed a process through which they hope to build trust with African regions. In just September, Burkina Faso has agreed to allow Target Malaria to release sterile mosquitoes in its communities. While gene drive is not being implemented in this approach, the organization hopes to begin building connections with the surrounding communities before implementing more drastic methods. To some, the idea that scientists can now eradicate a disease that has been tormenting populations for centuries seems unreal, yet the efficacy rates of the studies prove otherwise. The combination of CRISPR and gene drives may be the “abracadabra” those living in Ancient Rome so desperately desired, just a couple of centuries late.
