SENSORS INSIDE US
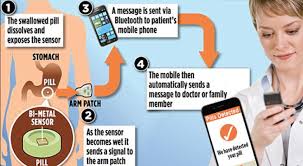
 BY ALEX HOLLOWAY – Tracking sensors are in almost all forms of technology, including cell phones, computers and cars, but now they can even be present in medications. Otsuka America Pharmaceutical, Inc. released the Abilify MyCite, which is an ingestible pill that contains a sensor inside. The sensor allows patients and their providers to record medication times, doses and activity levels through a skin patch and cell phone app. Although sensored pills are a innovative breakthrough, they have raised some troubling questions as well.
BY ALEX HOLLOWAY – Tracking sensors are in almost all forms of technology, including cell phones, computers and cars, but now they can even be present in medications. Otsuka America Pharmaceutical, Inc. released the Abilify MyCite, which is an ingestible pill that contains a sensor inside. The sensor allows patients and their providers to record medication times, doses and activity levels through a skin patch and cell phone app. Although sensored pills are a innovative breakthrough, they have raised some troubling questions as well.
In November of 2017, the FDA made Abilify MyCite the first drug in the U.S. with a digital ingestion tracking system. Its hope was to treat schizophrenia, bipolar one disorder and major depressive disorder in a more manageable and efficient way. After diagnosis and prescription, the process begins by downloading the MyCite app. After downloading, the user must stick on the skin patch which also contains a sensor for detecting signals from the medication and transmitting them to the cellphone app. Once the pill is ingested, it comes into contact with gastric fluid, magnesium and cuprous chloride within the sensor to power the device and communicate to the patch. The information that is transmitted into the app can be viewed not only by the patient, but can be monitored by a physician through a web based portal. Being able to track the dates and times that the medication is taken benefits patients and prescribers alike. For patients, there is the benefit of reassurance in knowing they took their medicine, and for physicians, they can ensure patients are staying on track with their care plan, while not having to make them pay or burden them with frequent visits to the office. The ability to manage patients medication consumption is particularly important in the case of an aripiprazole tablet, which is the drug located in the Abilify MyCite. The disorders which Abilify MyCite treats can have severe consequences when they are not managed properly and can even go to the extreme of death. With doctor’s ability to manage consumption closely, they can seek help for patients when necessary without overstepping boundaries.
Abilify MyCite could have made the difference in three children’s lives at a small home in South Georgia. Jacqueline Sanders of Albany, Ga was a 53 year old woman who was babysitting her three grandchildren one August afternoon. Sanders was a diagnosed schizophrenic, but she had been failing to take her prescription properly. Due to her lack of medication, Sanders’ condition caused her to fatally injure two of her grandchildren while one sustained stab wounds. Additionally, Sanders set her own home on fire where she succumbed to fatal injuries. Abilify MyCite hopes to eliminate similar situations through better medication management and education. Although intervening through an ingested sensor seems personal, it could prevent women like Sanders from forgetting to take their medicine which is all too common.
Convenience is a main selling point for Abilify MyCite in its ability to lessen doctor’s visits through increased social monitoring and communication. However, the elimination of face-to-face interaction that Abilify MyCite provides has raised some red flags in the healthcare community. While many people are intrigued by the sensored drug, they are also concerned it is going to continue to make doctor-patient relationships more distant. With distance, some argue patients are more likely to not adhere to their care plan. This belief stems from the idea that fewer doctor’s visits give patients less opportunities to open up about how they feel about their medications. Patients often voice that they have negative experiences with drug dosages and side effects but lack sufficient means of communication with their doctor to articulate and resolve this problem. Instead, it is thought that making frequent visits and receiving more in person interaction should be the core of mental health patients care plans.
With Abilify MyCite being relatively new, there is a plethora of information yet to be discovered. Regardless of good or bad, the sensored drug is a step toward a more technologically driven medical field and an enhancement in pharmaceuticals. It is exciting to see the success that Abilify MyCite has received by becoming FDA approved and to imagine the possibility of further advancement in the future.
